Abstract
Background: Clostridium difficile (CD) is an important cause of infectious diarrhea in patients with hematologic malignancies (HM) admitted to the hospital for chemotherapy or hematopoietic cell transplantation (HM-HCT patients). These patients are at increased risk of developing Clostridium difficile infection (CDI) compared to non-oncology patients, and they are more likely to suffer adverse outcomes from the infection. Asymptomatic colonization of patients with CD is known to precede infection. However, the reported prevalence of asymptomatic colonization varies widely between studies. The primary objective of this study was to define the prevalence of toxigenic CD colonization in patients with hematologic malignancies who were admitted to the hospital for scheduled chemotherapy or hematopoietic cell transplantation (HCT). The secondary objective was to determine what microbiology tests are accurate in detecting CD colonization compared to the gold standard of toxigenic culture.
Method: We performed a prospective cohort study of HM-HCT patients at our institution. Patients were included if they were age 18 years or older and scheduled for elective admission for chemotherapy or HCT (including both autologous and allogeneic HCT). Patients were excluded if they had diarrhea due to CDI in the past 30 days; if they had diarrhea of unknown etiology; if they were admitted on an unscheduled basis for treatment of acute illness; or if their anticipated length of stay was less than 72 hours. At the time of hospital admission for scheduled chemotherapy or HCT, a stool specimen was collected from each patient in a clean sterile collection vial. All samples were freshly collected and sent to the microbiology laboratory for processing. The stool samples were tested with six different methods: Simplexa™ C. difficile PCR Universal Direct Kit, Cepheid Xpert® PCR C.difficileCD/Epi (Xpert CD/Epi), C. diff Quik Chek Complete® GDH, C. diff Quik Chek Complete® Toxin A/B, LEUKO EZ VUE® ELISA test, and toxigenic culture. Toxigenic culture was used as the reference method. Baseline characteristics of those who tested positive versus negative were described using summary statistics, and univariable logistic regression analysis was used to identify risk factors for CD colonization. Sensitivity and specificity with 95% confidence interval for five tests were estimated.
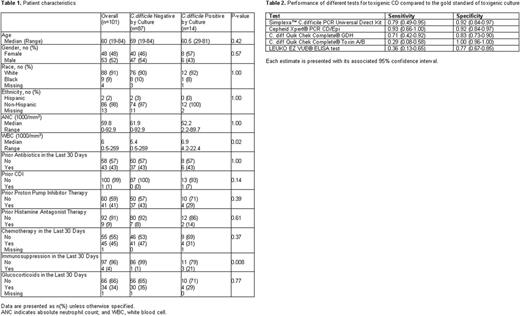
Results: A total of 114 patients were recruited for participation between April 2016 and November 2016. Thirteen patients either withdrew consent or did not provide a stool sample, so the final CD analysis included 101 patients. The demographic and clinical characteristics of the included patients are presented in Table 1. The prevalence of toxigenic CD colonization as determined by toxigenic culture was 13% (14/101, [95% CI: 8-22%]). The Cepheid Xpert PCR CD/Epi showed the highest sensitivity. The sensitivity and specificity of the different testing methods are shown in Table 2. The median (range) length of hospital stay was 13 (2-57) days. Among the 14 patients who tested positive by toxigenic culture, only 1 patient (7%) patient developed CDI during his hospitalization, and his stool tested positive by Cepheid Xpert PCR CD/Epi prior to the development of CDI. Univariable logistic regression analysis did not identify any risk factors for CD colonization including prior CDI, antibiotic use in the past 30 days, or treatment with proton pump inhibitors.
Conclusions: Toxigenic CD colonization was high in our cohort of patients, but the majority of patients who were colonized with toxigenic CD did not develop CDI while hospitalized. There were no identifiable risk factors for CD colonization. The most sensitive test for identifying toxigenic CD colonization was the Cepheid Xpert PCR CD/Epi test. Our findings suggest that the Cepheid Xpert PCR CD/Epi test could be used to screen for toxigenic CD colonization in patients admitted for scheduled chemotherapy or HCT. However, the costs and benefits of such an approach warrant further investigation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal